
- Team
- Project
- Lab
- Model
- Parts
- Improvement
- Contributions
- Judging

The lipid accumulation was also measured at 72 hours following dilution. In this time point, the culture had reached the stationary phase, and LDs were clearly detectable with Nile red staining for all tested strains, including the wild-type (Fig 2B). Lipid accumulation is known to increase in S. cerevisiae cells upon nutrient depletion (Werner-Washburne et al., 1993), and our results reveal that the difference between wild-type and TAG lipase deletion strains is considerably reduced in the stationary phase cells (Fig 2B). In the 72-hour time point, we did not observe a statistically significant difference between wild-type and the single tgl4Δ or double tgl3Δ tgl4Δ deletion cells. Single deletion of TGL3, which had the largest effect in the 24-hour time point, also led to increased LD staining intensity at 72 hours. We observed the highest intracellular lipid levels with the triple tgl3Δ tgl4Δ tgl5Δ deletion strain, and interestingly, in this time point, the zwf1Δ strain had considerably lower LD staining, comparable to the level of the wild-type strain (Fig 2B). Although the lipid levels of zwf1Δ strain were not lower in the 24-hour time point, ZWF1 deletion could be expected to result in decreased lipid synthesis, as Zwf1 is required to regenerate NADPH, a critical cofactor in fatty acid synthesis. Taken together, by preventing TAG degradation with triple deletion of TAG lipases, we have achieved a considerable increase in lipid production compared to the wild-type strain.
First, we s
The lipid accumulation was also measured at 72 hours following dilution. In this time point, the culture had reached the stationary phase, and LDs were clearly detectable with Nile red staining for all tested strains, including the wild-type (Fig 2B). Lipid accumulation is known to increase in S. cerevisiae cells upon nutrient depletion (Werner-Washburne et al., 1993), and our results reveal that the difference between wild-type and TAG lipase deletion strains is considerably reduced in the stationary phase cells (Fig 2B). In the 72-hour time point, we did not observe a statistically significant difference between wild-type and the single tgl4Δ or double tgl3Δ tgl4Δ deletion cells. Single deletion of TGL3, which had the largest effect in the 24-hour time point, also led to increased LD staining intensity at 72 hours. We observed the highest intracellular lipid levels with the triple tgl3Δ tgl4Δ tgl5Δ deletion strain, and interestingly, in this time point, the zwf1Δ strain had considerably lower LD staining, comparable to the level of the wild-type strain (Fig 2B). Although the lipid levels of zwf1Δ strain were not lower in the 24-hour time point, ZWF1 deletion could be expected to result in decreased lipid synthesis, as Zwf1 is required to regenerate NADPH, a critical cofactor in fatty acid synthesis. Taken together, by preventing TAG degradation with triple deletion of TAG lipases, we have achieved a considerable increase in lipid production compared to the wild-type strain.

Figure 1. Nile red staining shows build-up of lipids in LDs upon TAG lipase deletions. Microscopy images showing the cells in brightfield image and the fluorescent signal of lipids stained with Nile red. The cells are from 24 hour time point after inoculation.
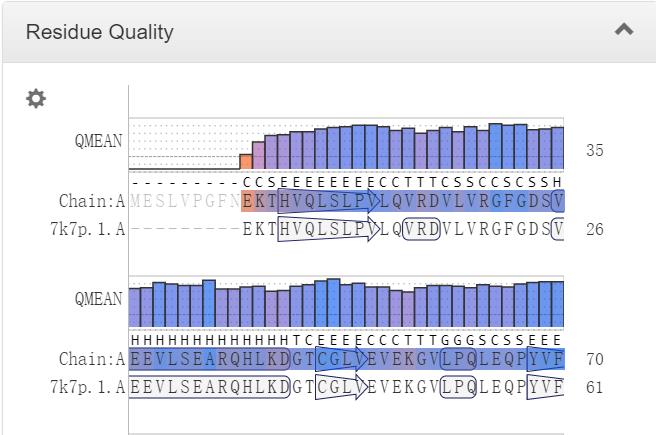
Figure 2. TAG lipase deletions result in greatly increased lipid accumulation in LDs. (A, B) Plots showing the mean Nile red fluorescence intensities in single cells from indicated strains. The samples were analyzed at 24 hour time point (A) and 72 hour time point (B). The bars show median and its 95% confidence intervals. ****, **, *, and ns note p-values <0.0001, <0.01, <0.05, and >0.05, respectively, of pair-wise comparisons with wild-type using Mann-Whitney U test.
The lipid accumulation was also measured at 72 hours following dilution. In this time point, the culture had reached the stationary phase, and LDs were clearly detectable with Nile red staining for all tested strains, including the wild-type (Fig 2B). Lipid accumulation is known to increase in S. cerevisiae cells upon nutrient depletion (Werner-Washburne et al., 1993), and our results reveal that the difference between wild-type and TAG lipase deletion strains is considerably reduced in the stationary phase cells (Fig 2B). In the 72-hour time point, we did not observe a statistically significant difference between wild-type and the single tgl4Δ or double tgl3Δ tgl4Δ deletion cells. Single deletion of TGL3, which had the largest effect in the 24-hour time point, also led to increased LD staining intensity at 72 hours. We observed the highest intracellular lipid levels with the triple tgl3Δ tgl4Δ tgl5Δ deletion strain, and interestingly, in this time point, the zwf1Δ strain had considerably lower LD staining, comparable to the level of the wild-type strain (Fig 2B). Although the lipid levels of zwf1Δ strain were not lower in the 24-hour time point, ZWF1 deletion could be expected to result in decreased lipid synthesis, as Zwf1 is required to regenerate NADPH, a critical cofactor in fatty acid synthesis. Taken together, by preventing TAG degradation with triple deletion of TAG lipases, we have achieved a considerable increase in lipid production compared to the wild-type strain.
The lipid accumulation was also measured at 72 hours following dilution. In this time point, the culture had reached the stationary phase, and LDs were clearly detectable with Nile red staining for all tested strains, including the wild-type (Fig 2B). Lipid accumulation is known to increase in S. cerevisiae cells upon nutrient depletion (Werner-Washburne et al., 1993), and our results reveal that the difference between wild-type and TAG lipase deletion strains is considerably reduced in the stationary phase cells (Fig 2B). In the 72-hour time point, we did not observe a statistically significant difference between wild-type and the single tgl4Δ or double tgl3Δ tgl4Δ deletion cells. Single deletion of TGL3, which had the largest effect in the 24-hour time point, also led to increased LD staining intensity at 72 hours. We observed the highest intracellular lipid levels with the triple tgl3Δ tgl4Δ tgl5Δ deletion strain, and interestingly, in this time point, the zwf1Δ strain had considerably lower LD staining, comparable to the level of the wild-type strain (Fig 2B). Although the lipid levels of zwf1Δ strain were not lower in the 24-hour time point, ZWF1 deletion could be expected to result in decreased lipid synthesis, as Zwf1 is required to regenerate NADPH, a critical cofactor in fatty acid synthesis. Taken together, by preventing TAG degradation with triple deletion of TAG lipases, we have achieved a considerable increase in lipid production compared to the wild-type strain.